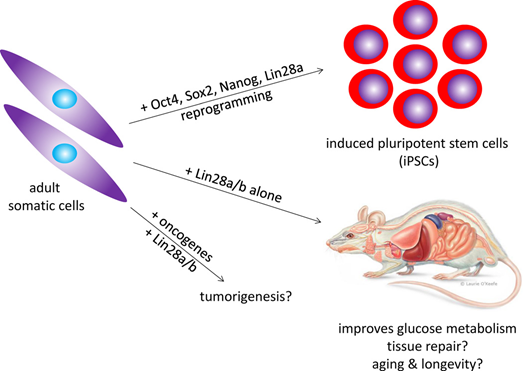
The Lin28/Let7 pathway was originally described as a regulator of developmental timing in C elegans, but more recently we have linked this ancient pathway to stem cells and cancer biology. Lin28 is an RNA-binding protein that regulates gene expression via two different mechanisms: one that blocks Let7 biogenesis and another that involves direct binding to a wide array of mRNA targets. The Let7 family of miRNAs regulates many factors that control cell-fate decision, including oncogenes (c-myc, Ras, HMGA-2) and cell-cycle factors (CyclinD1, D2). In mammals, Lin28A and its closely related paralog Lin28B are highly expressed in pluripotent cells, where they play an important role in the maintenance of self-renewal and proliferation. Both Lin28 proteins are highly expressed in early embryonic development but become down-regulated over time, while levels of mature Let7 famlily members rise as stem cells differentiate into specialized tissue types.
We have engineered several murine strains that overexpress or are deficient in Lin28 and have observed remarkable phenotypes that implicate the Lin28/Let7 axis in germ cell development, tissue regeneration, regulation of human growth and developmental timing, glucose metabolism, predisposition to diabetes and cancer. Thus, we suggest that this primal pathway evolved to balance cell proliferation and the metabolic needs of growing tissues and organisms. Our future studies will aim at exploring modes of drug targeting of this medically important pathway for treatment of cancer and metabolic diseases.
Selected Publications
Lin28b Is Sufficient to Drive Liver Cancer and Necessary for Its Maintenance in Murine Models. Liem H. Nguyen, Daisy A. Robinton, Marc T. Seligson, Linwei Wu, Lin Li, Dinesh Rakheja, Sarah A. Comerford, Saleh Ramezani, Xiankai Sun, Monisha S. Parikh, Erin H. Yang, John T. Powers, Gen Shinoda, Samar P. Shah, Robert E. Hammer, George Q. Daley, Hao Zhu. Cancer Cell 2014 August 11:26(2): 248-61
Lin28 sustains early renal progenitors and induces Wilms tumor. Urbach A, Yermalovich A, Zhang J, Spina CS, Zhu H, Perez-Atayde AR, Shukrun R, Charlton J, Sebire N, Mifsud W, Dekel B, Pritchard-Jones K, Daley GQ. Genes Dev. 2014 May 1;28(9):971-82.
Lin28 enhances tissue repair by reprogramming cellular metabolism. Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ. Cell. 2013 Nov 7;155(4):778-92.
Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Shinoda G, Shyh-Chang N, Soysa TY, Zhu H, Seligson MT, Shah SP, Abo-Sido N, Yabuuchi A, Hagan JP, Gregory RI, Asara JM, Cantley LC, Moss EG, Daley GQ. Stem Cells. 2013 Aug;31(8):1563-73.
Lin28: primal regulator of growth and metabolism in stem cells. Shyh-Chang N, Daley GQ. Cell Stem Cell. 2013 Apr 4;12(4):395-406.
The Lin28/let-7 axis regulates glucose metabolism. Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI; DIAGRAM Consortium; MAGIC Investigators, Altshuler D, Daley GQ. Cell. 2011 Sep 30;147(1):81-94.
Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ. Nat Genet. 2010 Jul;42(7):626-30.
Lin28: A microRNA regulator with a macro role. Viswanathan SR, Daley GQ. Cell. 2010 Feb;140(4):445-9.
A role for Lin28 in primordial germ-cell development and germ-cell malignancy. West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL, Surani MA, Daley GQ. Nature. 2009 Aug 13;460(7257):909-13.
Lin28 promotes transformation and is associated with advanced human malignancies. Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, Mermel CH, Beroukhim R, Azam M, Teixeira J, Meyerson M, Hughes TP, Llovet JM, Radich J, Mullighan CG, Golub TR, Sorensen PH, Daley GQ. Nat Genet. 2009 Jul;41(7):843-8.
Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, Gregory RI. J Biol Chem. 2008 Aug 1;283(31):21310-4.
Selective blockade of microRNA processing by Lin28. Viswanathan SR, Daley GQ, Gregory RI. Science. 2008 Apr 4;320(5872):97-100.
